What Does Cholesterol Do In Animal Cell Membranes
- Research
- Open Access
- Published:
Cholesterol content in cell membrane maintains surface levels of ErbB2 and confers a therapeutic vulnerability in ErbB2-positive breast cancer
Cell Communication and Signaling volume 17, Article number:fifteen (2019) Cite this article
Abstract
Background
ErbB2 overexpression identifies a subset of breast cancer as ErbB2-positive and is frequently associated with poor clinical outcomes. As a membrane-embedded receptor tyrosine kinase, cell surface levels of ErbB2 are regulated dynamically by membrane physical properties. The present study aims to investigate the influence of membrane cholesterol contents on ErbB2 status and cellular responses to its tyrosine kinase inhibitors.
Methods
The cholesterol abundance was examined in ErbB2-positive breast cancer cells using filipin staining. Cellular ErbB2 localizations were investigated by immunofluorescence with contradistinct membrane cholesterol contents. The inhibitory effects of the cholesterol-lowering drug lovastatin were assessed using cell proliferation, apoptosis, immunoblotting and immunofluorescence assays. The synergistic effects of lovastatin with the ErbB2 inhibitor lapatinib were evaluated using an ErbB2-positive breast cancer xenograft mouse model.
Results
Membrane cholesterol contents positively correlated with cell surface distribution of ErbB2 through increasing the rigidity and decreasing the fluidity of cell membranes. Reduction in cholesterol abundance assisted the internalization and degradation of ErbB2. The cholesterol-lowering drug lovastatin significantly potentiated the inhibitory furnishings of ErbB2 kinase inhibitors, accompanied with enhanced ErbB2 endocytosis. Lovastatin also synergized with lapatinib to strongly suppress the in vivo growth of ErbB2-positive breast cancer xenografts.
Conclusion
The cell surface distribution of ErbB2 was closely regulated by membrane physical properties governed past cholesterol contents. The cholesterol-lowering medications tin hence be exploited for potential combinatorial therapies with ErbB2 kinase inhibitors in the clinical treatment of ErbB2-positive breast cancer.
Groundwork
ErbB2, which is as well called Her2 or Neu, encodes a receptor tyrosine kinase from the EGFR/ErbB family. Unlike other ErbB members, ErbB2 has no identified cognate ligand, and thus till now is accounted every bit an orphan receptor [1]. Nevertheless, due to the unique conformation of its extracellular domain, this receptor tyrosine kinase is considered equally a favorable dimerization partner among ErbB family unit members [2, 3]. Amplification of ErbB2 cistron is frequently observed in cancer patients, which identifies a subgroup of breast cancers called Her2/ErbB2-positive that accounts for 20–thirty% of chest malignancies. ErbB2 amplification leads to the aggregating of surplus ErbB2 receptors on cell membrane, promoting receptor dimerization and subsequent activation of a wide array of downstream oncogenic signaling circuitries [4, 5]. Hence, the overexpression of ErbB2 inversely correlates with patient prognosis, while ErbB2 has proved every bit a tiptop therapeutic target in breast cancer treatment with multiple ErbB2-targeted therapies received FDA approvals [6,seven,8].
ErbB2 is a single laissez passer transmembrane receptor embedded in the plasma membrane, a complex structure composed of primarily lipids and proteins [9,10,11]. Amid its many essential physiological functions, cell membrane plays an of import office to maintain the homeodynamics of jail cell surface proteins including the receptor tyrosine kinase ErbB2 [12,13,14]. On average, about half of the weight of eukaryotic plasma membranes tin can exist attributed to lipids, which form the bilayer membrane structures incorporating three types of amphipathic lipids: phospholipids, sterols, and glycolipids [15, xvi]. The bulk of the lipid bilayer is equanimous of phospholipids and sterols, while glycolipids only make up a small fraction of less than 5% in general.
Cholesterol is the major sterol component of animal cell membranes, which makes upward about thirty% of the lipid bilayer on average. Interim as essential edifice blocks of the plasma membranes, cholesterol plays pivotal roles in maintaining the structural integrity and regulating the fluidity of prison cell membranes [17,xviii,19,20], therefore contributing to the homeodynamics of various membrane proteins on the cell surface. For example, alterations in membrane microviscosity and lipid fluidity mediated past cholesterol depletion or enrichment were revealed to significantly affect the cell surface distribution of membrane proteins in human erythrocytes [21, 22]. Furthermore, regarding its cell membrane-associated functions, cholesterol is besides implicated in the modulation of cellular bespeak transmission and intracellular trafficking through contributing to lipid raft assembly and assisting the germination of endocytic pits [23, 24]. Although the oncogenic properties of ErbB2 in breast cancer has been extensively investigated, the connexion between its expression levels and the concrete properties of chest cancer cell membranes is obscure. Several proteins including HSP90, flotillin, and caveolin have been shown to regulate the jail cell surface distribution of ErbB2, only how cholesterol content in cell membrane regulates the overall surface presence of this cancer-driving receptor tyrosine kinase remains elusive so far [25,26,27,28]. In the nowadays report, we study that cholesterol content modulates the rigidity and fluidity of plasma membranes to maintain the surface levels of ErbB2 in breast cancer cells, while the reduction in cholesterol abundance in plasma membrane facilitates the endocytic degradation of ErbB2 and thus synergizes with the tyrosine kinase inhibitors against ErbB2 to suppress ErbB2-positive breast cancer growth.
Methods
Cell lines
Breast cancer SKBR3, AU565, and HCC1954 jail cell lines were purchased from the American Type Culture Drove (ATCC). SKBR3 cells were cultured with McCoy's 5A, while AU565 and HCC1954 cells were cultured with RPMI-1640 media, both supplemented with fetal bovine serum (ten%, ExCell Bio, Shanghai) and antibiotics (1% penicillin/streptomycin, Gibco). Cells were maintained in a humidified atmosphere in the incubator (Thermo) at 37 °C with 5% CO2.
Antibodies and other reagents
Mouse anti-ErbB2 (A-2), anti-ErbB2 (9G6), anti-Vinculin antibodies were purchased from Santa Cruz Biotechnology (CA, Usa). Rabbit anti-PARP antibody was purchased from Proteintech (Wuhan, China). Rabbit anti-phospho-Akt (Ser473) antibiotic was purchased from Cell Signaling Engineering science. Secondary goat anti-mouse and anti-rabbit, donkey anti-goat antibodies were obtained from LICOR. Neratinib (HKI-272) and lapatinib (GW572016) were purchased from Selleck. Oleic acid (OA) and lovastatin were obtained from MeilunBio (Dalian, Communist china). Filipin was obtained from Sigma.
Jail cell lysis and immunoblottings
Cells were lysed with the RIPA buffer (10 mM Tris-HCl pH seven.5, 150 mM NaCl, 1% (w/five) TritonX-100, 0.i% (west/5) SDS, 1% sodium deoxycholate) supplemented with mammalian protease and phosphatase inhibitor cocktails (Sigma) as described previously [29]. Protein concentrations were determined via BCA protein assays (Takara). In general, 20–30 μg of protein samples were loaded per lane onto SDS-Page gels to be resolved, earlier transferred to nitrocellulose membranes (Merck Millipore, USA). Membranes were then blocked with 4% not-fat-milk in PBS at room temperature for an hour, followed by incubation with master antibodies at 4 °C overnight. The blots were washed with PBS for 3 times before incubation with secondary antibodies. Membranes were finally washed with PBS and detected on a LICOR Odyssey system. Captured images were quantitated using Image Studio software (Version four.0) every bit per manufacturer's instructions.
MTT assay
Two thousand breast cancer cells were seeded into each well of 96-well plate and incubated overnight. Next day, cells were treated with indicated inhibitors for 24 h, before MTT (3-(four, 5-dimethylthiazol-2-yl)-2,five diphenyltetrazolium bromide) incubation for another 3 h. DMSO was added to dissolve formazan and absorbance was recorded at 570 nm using a spectrometer (Perkin Elmer, USA).
Wound healing assay
Cells were seeded onto 6-well plates to form a confluent monolayer. A wound was generated by scratching with a 200 μl pipette tip. Discrete cells were cleaned by PBS washes. Mitomycin was added into growth media to a final concentration of 2.v μg/ml to terminate cell proliferation. Images of wound recovery were captured every 24 h using a phase contrast microscope (Leica, DMI4000B).
Flow cytometry
For apoptosis assays, cultured cells were processed for Annexin V and PI double staining using an apoptosis analysis kit (KeyGEN Biotech, People's republic of china) co-ordinate to manufacturer's instructions. The samples were finally analyzed using a benchtop menstruation cytometer (Accuri C6, BD Biosciences) and acquired data were analyzed using FlowJo software version 7.6.1 (FlowJo, LLC, USA).
Cholesterol staining
AU565, SKBR3, and HCC1954 cells were seeded onto coverslips placed in six-well plates with total media. Next twenty-four hour period, cells were washed with PBS for 3 times and fixed with four% paraformaldehyde for 15 min at room temperature. Following PBS washes, cells were incubated with 50 μg/ml of filipin for one h at room temperature, equally described previously [30]. Then coverslips were washed and mounted onto a slide using Mowiol (Sigma). Cholesterol staining was examined with ultraviolet excitation using a fluorescence microscope (Olympus, BX63).
Immunofluorescence
Cultured SKBR3, AU565, and HCC1954 cells were treated every bit indicated. After PBS washes, cells were fixed with 4% paraformaldehyde for xv min and so permeabilized with 0.2% Triton X100. Samples were blocked with ii% BSA in PBS for half an hr, before incubation with primary antibodies for 20 min at room temperature. Coverslips were washed with PBS for iii times and incubated with Alexa Fluor® 488-conjugated goat anti-mouse secondary antibodies for 20 min at room temperature. Finally coverslips were washed and mounted onto a slide using Mowiol supplemented with DAPI (for visualization of cell nucleus). Antibiotic staining was observed under a fluorescence microscope (Olympus, BX63).
Xenograft mouse model
Animal experiments were performed in accord with the 1996 National Institutes of Health Guide for the Care and utilise of Laboratory Animals, and the procedures were approved by the Institutional Animal Intendance and Use Commission of Dalian Medical Academy. Female nude mice (iv–6 weeks, Balb/c groundwork) were obtained from Vital River Laboratories (Beijing, China) and maintained nether sterile conditions during the unabridged experiments. To generate tumor xenografts, cultured HCC1954 cells (6 million/mouse) were implanted subcutaneously into the right flank of each mouse. The sizes of the xenograft tumors were measured using a vernier caliper every 3 days. After the average size of xenografts reached about 150 mm3, the mice were randomly divided into iii groups (5 mice per group) to receive lapatinib, lovastatin and lapatinib, or vehicle control treatment. Lapatinib was dissolved in 2% DMSO, 30% PEG 300, and 5% Tween fourscore in distilled h2o, while lovastatin was dissolved in thirty% PEG 400, 0.5% Tween 80, and 5% propylene glycol in distilled water. Lapatinib and lovastatin were administered by daily oral gavage for 17 consecutive days at dosages of 100 mg/kg and xl mg/kg, respectively. Tumor volumes were calculated according to the following formula: tumor volume (mm3) = (length) × (width)2 × 0.52. Tumor xenografts were finally excised from the mice after sacrifice, and processed for Western blotting analyses.
Statistics
To determine statistical significance, experiments were conducted three times with biological repeats. Experimental results were presented every bit the means ± standard error of the mean (SEM). Statistical differences between experimental groups were examined by performing Student's t-exam in the GraphPad Prism software (version 7), and a p value less than 0.05 was considered every bit statistically significant.
Results
Cholesterol content in cell membrane correlates with ErbB2 localization and cell migration
Through immunofluorescence examination of ErbB2 localization in ErbB2-positive breast cancer cells, we observed that, in SKBR3 and AU565 cells that possessed circular shapes, ErbB2 was almost exclusively distributed to the cell membrane; while in HCC1954 cells that showed flattened and all-inclusive configurations, ErbB2 likewise formed many intracellular punctae besides surface localizations (Fig. 1a). This influence of jail cell shapes on ErbB2 distribution prompted us to speculate that the physical properties of prison cell membranes might play a role in the regulation of subcellular distribution of ErbB2, i.eastward. the round and more rigid jail cell surfaces in SKBR3 and AU565 cells tended to maintain ErbB2 in the cell membrane, while the floppy and more fluid cell membrane of HCC1954 facilitated the internalization of this receptor. Considering the essential roles of cholesterol in regulating cell membrane rigidity and fluidity [31, 32], nosotros examined the cholesterol content in prison cell membranes from these three ErbB2-positive breast cancer prison cell lines. Results from fluorescence microscopy using the cholesterol-specific stain filipin revealed that the cell membrane cholesterol in SKBR3 and AU565 cells was considerably more abundant than that in HCC1954 cells (Fig. 1b).
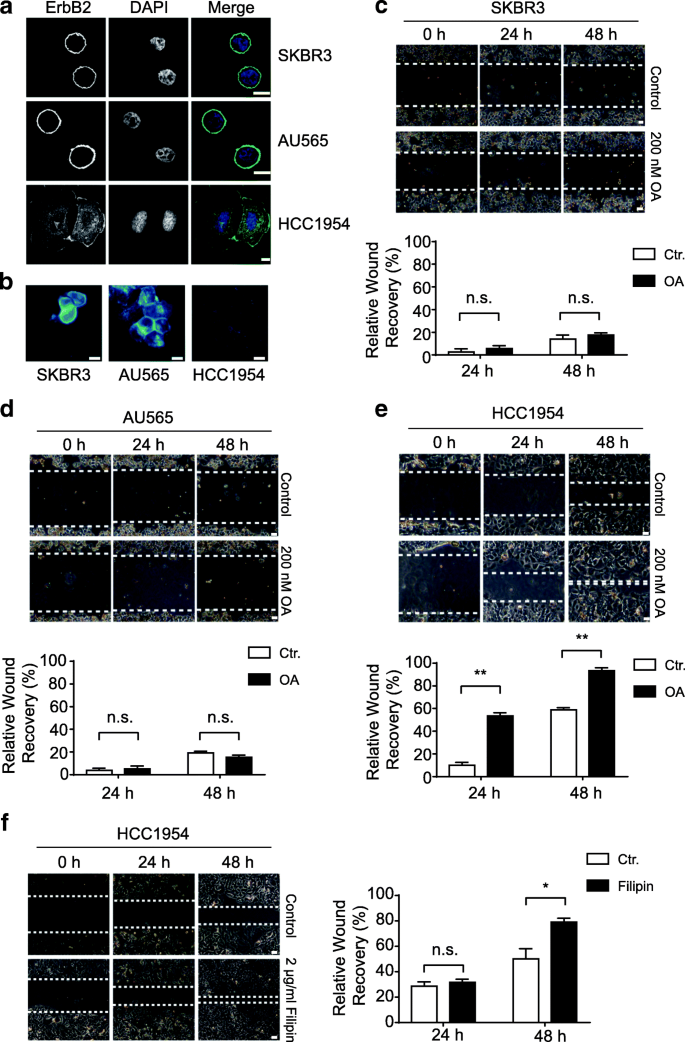
Prison cell membrane cholesterol content correlates with ErbB2 localization and prison cell migration in ErbB2-positive breast cancer cells. a ErbB2-positive SKBR3, AU565, and HCC1954 cells were cultured on glass coverslips and candy for immunofluorescence analyses with anti-ErbB2 antibody. Nucleus was stained with DAPI. Representative images show confocal sections. Scale bar = 10 μm. b indicated cells were cultured and processed for filipin staining to examine cholesterol amounts. Fluorescence images were captured nether identical intensity settings. Scale bar = 10 μm. c, d, e and f, indicated chest cancer cells were grown to grade confluent monolayers and processed for wound healing assays. Mitomycin (2.5 μg/ml) was added during recovery. Cells were either treated with DMSO (control), oleic acid (OA) at 200 nM, or filipin at 2 μg/ml. Images of the wounds were recorded at indicated times under a phase dissimilarity microscope. Scale bar = 50 μm. Adjacent cavalcade charts bear witness the quantification of relative wound recovery from each condition. Not significant, n.s., * and ** indicate p < 0.05 and p < 0.01, respectively
As cellular cholesterol levels were shown to regulate metastatic phenotypes of cancer cells past altering the fluidity of cell membranes, nosotros investigated whether the differential cholesterol amounts in ErbB2-positive breast cancer cells correlated with their migratory abilities [33]. Indeed, very limited cell migration was observed in SKBR3 and AU565 cells bearing high cholesterol contents, indicative of increased rigidity and reduced fluidity of cell membranes (Fig. 1c and d). Even in the presence of oleic acid that could increase membrane fluidity to some extent, the migration of SKBR3 and AU565 cells was however restricted, likely owing to the relatively high rigidity and low fluidity of the plasma membranes incurred by the abundance of cholesterol (Fig. 1c and d). On the contrary, in HCC1954 cells that independent relatively low levels of cholesterol, cell migration was readily observable, which was further enhanced by treatments with oleic acrid or the cholesterol-interfering drug filipin, suggesting that increased membrane fluidity with reduced cholesterol content in prison cell membrane assisted cell migration (Fig. 1e and f).
Cholesterol abundance maintains ErbB2 levels on cell surface
To further investigate the influence of cholesterol content on the intracellular distribution of ErbB2, we examined the localizations of this receptor in SKBR3, AU565, and HCC1954 cells exposed to the cholesterol-interfering drug filipin. Interestingly, the usually round-shaped SKBR3 and AU565 became more flattened under filipin treatment, and intracellular ErbB2 punctae were readily discernible, suggesting that filipin handling induced receptor internalization (Fig. 2a and b). In add-on, nosotros treated SKBR3 and AU565 cells with the combination of oleic acrid and filipin to further increase membrane fluidity and reduce surface rigidity. In this scenario, both SKBR3 and AU565 cells became more all-inclusive and irregular-shaped, while intracellular ErbB2 staining was greatly enhanced (Fig. 2a and b). On the other hand, although ErbB2 internalization was already evident in HCC1954 cells under normal culturing atmospheric condition, the addition of filipin and oleic acrid significantly increased the germination of intracellular ErbB2 punctae (Fig. 2c). Taken together, these observations signal that the cholesterol content in prison cell membrane regulates membrane rigidity and fluidity to maintain ErbB2 receptors on the cell surface, while the decrease of cholesterol abundance in plasma membrane leads to reduced rigidity and increased fluidity of cell membrane that assist ErbB2 internalization.
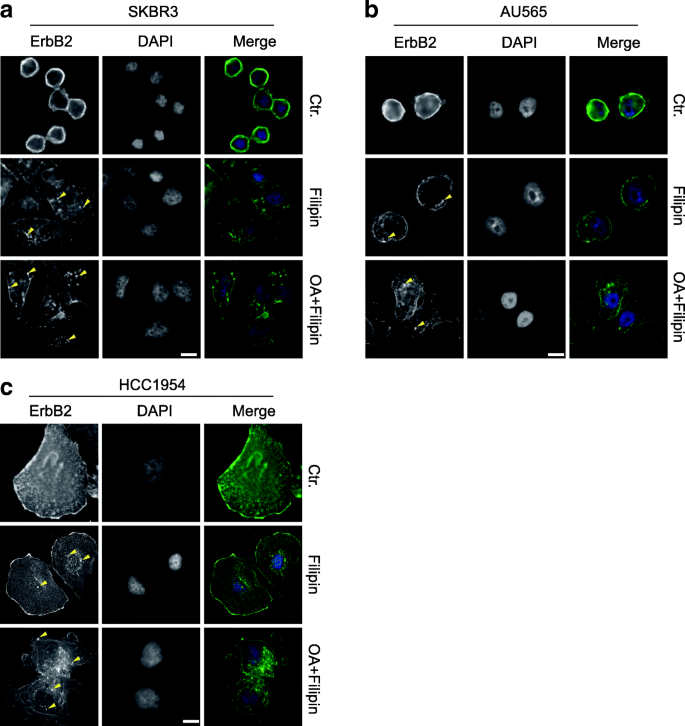
The cholesterol-interfering drug filipin facilitates ErbB2 internalization. a, b and c, ErbB2-positive SKBR3, AU565, and HCC1954 cells were cultured on glass coverslips and treated with filipin (3 μg/ml) with or without oleic acid (OA, 200 nM) for 12 h. ErbB2 location was examined by immunofluorescence. DAPI stains the nucleus. Images were captured using a fluorescent microscope (Olympus BX63, 40X objective). Representative intracellular ErbB2 punctae are indicated with yellow triangles. Calibration bar = 10 μm
Cholesterol-lowering drug potentiates ErbB2-targeting agents to suppress breast cancer growth
Having disclosed the correlation between cholesterol content in the cell membrane and the surface levels of ErbB2 receptors, we next sought to investigate the influence of the cholesterol-lowering drug lovastatin on the in vitro growth of ErbB2-positive breast cancer cell lines. In doing this, breast cancer cells overexpressing ErbB2 (SKBR3, AU565, and HCC1954) were treated with lovastatin, the FDA-canonical tyrosine kinase inhibitors against ErbB2 (lapatinib and neratinib), or the combinations every bit indicated (Fig. 3a). Results from cell viability assays revealed that lovastatin significantly inhibited cell growth, although less potent than lapatinib and neratinib, just besides potentiated the suppression incurred past these two tyrosine kinase inhibitors (Fig. 3a). Furthermore, flow cytometric data from Annexin 5 and propidium iodide double staining showed that the addition of lovastatin dramatically enhanced the pro-apoptotic effects of the ErbB2 inhibitors, with increases on averages of 3.03 and two.74 folds in SKBR3, 2.26 and 1.99 folds in AU565, and 1.seven and 1.75 folds in HCC1954 cells observed for lapatinib and neratinib, respectively (Fig. 3b and c). Therefore, these findings suggest that the cholesterol-lowering drug lovastatin is capable of sensitizing the ErbB2-positive chest cancer cells to both lapatinib and neratinib.
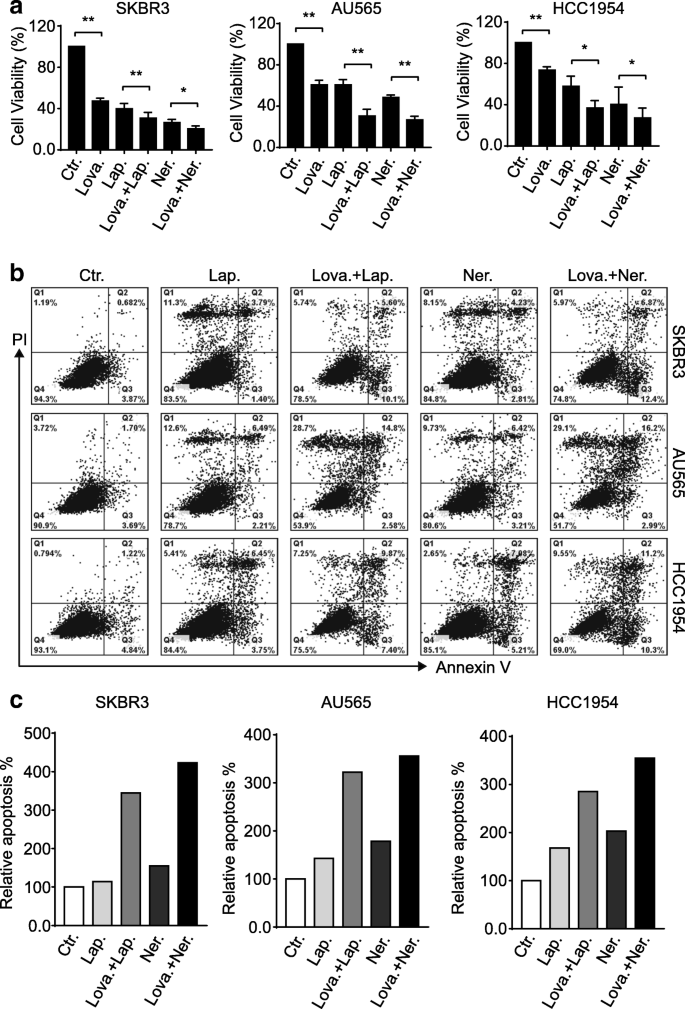
Lovastatin potentiates ErbB2 kinase inhibitors to suppress ErbB2-positive chest cancer cells. a SKBR3, AU565, and HCC1954 cells were treated with lovastatin (lova, 40 μM), lapatinib (lap, 200 nM), neratinib (ner, 200 nM), or combinations equally indicated for 24 h. Cell viability was then examined via MTT assays. Cavalcade charts show data from three independent experiments and error bars represent the standard error of the mean (SEM), with * and ** indicating p < 0.05 and p < 0.01, respectively. b breast cancer cells were treated as above and processed for double staining with Annexin V and propidium iodide (PI) to inspect apoptotic populations. c cavalcade charts show the quantitation of apoptotic cells with positive Annexin V staining (right ii quadrants) from b
Lovastatin treatment leads to increased endocytic deposition of ErbB2
Given that cholesterol abundance in the cell membrane could maintain the surface levels of ErbB2, we speculated that the inhibitory effects of the cholesterol-lowering drug lovastatin confronting ErbB2-positive breast cancer cells might be associated with compromised cell membrane expression of ErbB2 and the resultant attenuation of downstream point transmission. Every bit expected, results from immunoblotting assays revealed that lovastatin handling led to reduced ErbB2 expression in serum-starved SKBR3, AU565, and HCC1954 cells (Fig. 4a). In accord with our previous findings, treatments with the ErbB2 inhibitors lapatinib and neratinib resulted in increased and decreased cellular ErbB2 levels, respectively [34]. All the same, lovastatin add-on caused meaning reductions in ErbB2 expression nether the combined weather condition compared to inhibitor single treatments, with concomitantly enhanced PARP cleavage and decreased activation of AKT (Fig. 4a). Consistently, in the subsequent immunofluorescence assays to examine the cellular localizations of ErbB2, elevated intracellular ErbB2 staining was readily detected in HCC1954 cells treated with lovastatin (Fig. 4b and c). Fifty-fifty in neratinib-treated cells where ErbB2 endocytosis was induced, the combined treatment with lovastatin further augmented the formation of intracellular ErbB2 punctae (Fig. 4b and c). These immunoblotting and immunofluorescence data collectively suggest that lovastatin facilitated the internalization and intracellular deposition of ErbB2, leading to reduced ErbB2 expression levels and repressed downstream oncogenic signaling.
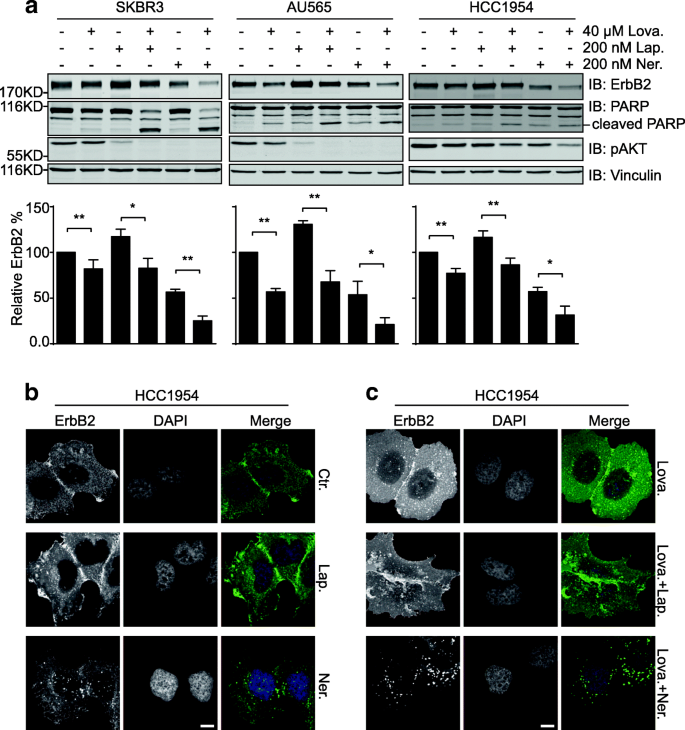
Lovastatin addition results in enhanced internalization and degradation of ErbB2. a SKBR3, AU565, and HCC1954 cells were treated with lovastatin (40 μM), lapatinib (200 nM), neratinib (200 nM), or combinations as indicated for 24 h. Jail cell lysates were analyzed by immunoblotting with indicated antibodies. Cavalcade graphs below testify the quantification of relative ErbB2 affluence compared to control samples. All fault bars represent the standard error of the mean (n = iii), with * and ** indicating p < 0.05 and p < 0.01, respectively. b HCC1954 cells were treated with lapatinib (200 nM) or neratinib (200 nM) earlier immunofluorescence analysis to inspect ErbB2 staining. DAPI stains the nucleus. Scale bar = 10 μm. Images were captured using a fluorescent microscope (Olympus BX63, 40X objective). c cultured HCC1954 cells were treated with lovastatin (40 μM) either lone or in the presence of lapatinib or neratinib (both at 200 nM). Immunofluorescence assays were then carried out as in b
Lovastatin synergizes with lapatinib to suppress the in vivo growth of ErbB2-positive chest cancer
Although nearly all types of animal cells generate cholesterol, the bulk (80%) of cholesterol synthesis at the organism level is accomplished in the liver and intestines. To investigate the synergistic effects of ErbB2 inhibitor and cholesterol-lowering drug in vivo, we generated ErbB2-positive breast cancer xenograft mouse models past implanting HCC1954 cells into athymic nude mice. Considering that lapatinib is more than widely administered than neratinib in the clinical treatment of ErbB2-positive breast cancer, nosotros treated the xenograft-bearing mice with lapatinib or the combination of lapatinib and lovastatin. As illustrated in Fig. 5a-c, lapatinib significantly suppressed HCC1954 xenograft growth in vivo, while the combo handling with lapatinib and lovastatin further decreased the sizes and weights of xenograft tumors formed in athymic nude mice, suggesting a synergistic effect of lovastatin with lapatinib to restrain the in vivo growth of ErbB2-positive breast cancer. Later on, we examined the expression levels of ErbB2 in xenograft tissues resected from mice with different treatments using immunoblotting assays. Consistent with our data from cell line studies, the ErbB2 levels were observed to be elevated in the samples from lapatinib treatment grouping compared to those of control group; while the combined treatment with lovastatin and lapatinib impeded the lapatinib-induced upregulation of ErbB2, confirming the negative regulatory effect of lovastatin on ErbB2 expression (Fig. 5d).
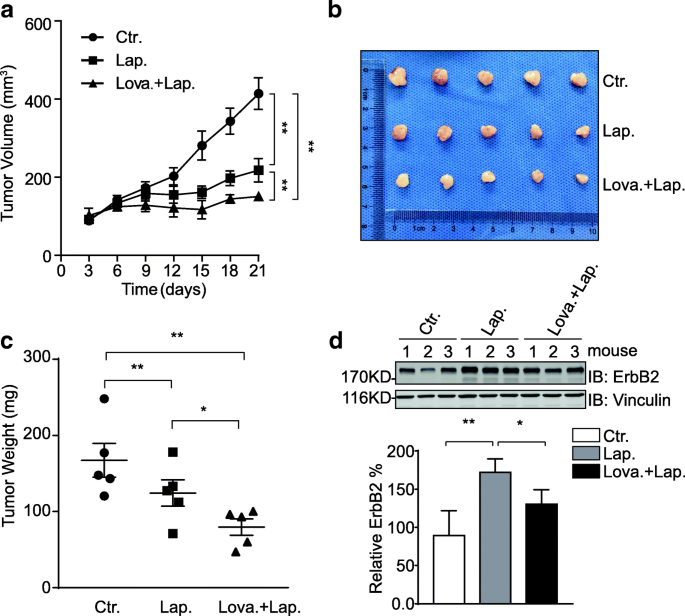
Lovastatin synergizes with lapatinib to suppress HCC1954 xenograft growth. a HCC1954 cells were inoculated into female nude mice to generate xenograft mouse models. Mice were randomized into 3 groups to receive control vehicle, lapatinib, or the combination of lovastatin and lapatinib equally described in the methods section. The sizes of xenografts were measured every 3 days and calculated tumor volumes were plotted. Presented graph shows data from 5 mice per grouping. Error bars show the standard mistake of the mean (SEM), with ** indicating p < 0.01. b after 17 days of oral gavage handling, the mice were sacrificed and xenograft tumors were resected. c tumor weight was measured and plotted. Presented graph shows data from v mice per group. Error confined evidence the standard error of the mean (SEM), with * and ** indicating p < 0.05 and p < 0.01, respectively. d protein samples were prepared from xenograft tumor tissues (3 per treatment group) and analyzed by immunoblotting using indicated antibodies. Vinculin was probed to show equal loading. Bar nautical chart below shows the relative quantification of ErbB2
Discussion
Chest cancer occurs with the highest incidence among all women malignancies and accounts for near 30% of newly diagnosed women cancer cases annually in the The states [35]. This leading deadly affliction is generally divided into luminal A, luminal B, HER2/ErbB2-positive, and triple-negative breast cancer subtypes according to the expression status of estrogen receptor, progesterone receptor, and HER2/ErbB2 [36]. ErbB2 overexpression in breast cancer is always associated with poor patient prognosis [37, 38], but ErbB2 targeted therapies accept significantly improved patient survivals, hence confirming this receptor tyrosine kinase every bit the pivotal therapeutic target for intervention in the treatment of ErbB2-positive breast cancers.
Through the conscientious observations of singled-out patterns in ErbB2 distribution of ErbB2-positive breast cancer cells, we found a correlation betwixt cell shape and ErbB2 localizations wherein ErbB2 remained mainly on the surface in cells with circular shapes but became internalized in those with irregular forms. We speculated that the physical features of jail cell membranes, including rigidity and fluidity, might play a role to influence the cellular ErbB2 localizations. It was therefore intriguing to find the remarkable deviation in cholesterol contents between SKBR3/AU565 and HCC1954 cells. Cholesterol is a major component of animal cell membranes, which is required to maintain the integrity and regulate the fluidity of plasma membranes [23]. Cholesterol contains a tetracyclic band structure and adopts a trans conformation, rendering the molecule a planar and rigid characteristics that increases membrane packing and contributes to the integrity and rigidity of cell membranes. Cholesterol is also a key determinant of membrane fluidity: at high temperatures, cholesterol acts to stabilize the cell membrane and increase its melting point; while at low temperatures, it inserts into phospholipids and prevents them from interfering with each other to avert aggregation [39]. Consistent with our hypothesis, the cholesterol abundance conferred the cell membranes of SKBR3 and AU565 cells increased rigidity and reduced fluidity that collectively express prison cell mobility. In add-on, by manipulating the cholesterol content in prison cell membranes using oleic acid and the cholesterol-interfering drug filipin, the cell shapes of SKBR3 and AU565 could exist effectively altered and subsequent ErbB2 internalization was apparently enhanced.
These findings inspired us to investigate the therapeutic effects of cholesterol lowering drugs from the statin course of the HMG-CoA reductase inhibitors, such every bit lovastatin, on the in vivo and in vitro growth of ErbB2-positive breast cancers. Through a series of cell phenotypic experiments including prison cell viability and apoptosis assays, we demonstrated that lovastatin significantly potentiated the inhibitory effects of the FDA-approved ErbB2 kinase inhibitors, lapatinib and neratinib. From the mechanistic attribute, we revealed that lovastatin add-on upon lapatinib and neratinib handling led to reduced cellular ErbB2 levels compared to inhibitor monotherapies, likely due to elevated internalization and degradation of ErbB2 incurred by altered membrane physical properties. More than importantly, using ErbB2-positive chest cancer xenograft mouse models, nosotros confirmed the stiff synergistic furnishings of lovastatin and lapatinib to suppress the in vivo growth of HCC1954 xenografts.
It is noteworthy that several statins that received FDA approvals as lipid-lowering medications have been reported to suppress breast cancer growth. For case, simvastatin was shown to induce PTEN transcription by interfering with NFκB activeness to inhibit the proliferation of chest cancer cells [forty]. This statin was also observed to inhibit the mammosphere formation and migration of triple-negative breast cancer cells through the regulation of FOXO3a [41]. Lovastatin was reported to suppress the tumor growth and metastasis in a mouse chest cancer animal model by causing increased apoptosis and decreased DNA synthesis [42]. Lovastatin was also shown to suppress the glycolytic and citric acid cycle activity to inhibit the proliferation of breast cancer MDAMB231 and MDAMB468 cells [43]. Furthermore, a contempo study proposed that lovastatin in a cerasome-encapsulated nanohybrid form effectively suppressed the stemness properties of triple-negative chest cancer [44]. In addition, pitavastatin was recently reported to sensitize chest cancer to radiation by delaying Deoxyribonucleic acid Repair and promoting senescence [45]. Therefore, information technology seemed that the statin class of medications exhibited pleiotropic effects to suppress the growth of breast cancer, while our findings revealed a novel attribute of the anti-cancer properties of the statins. Lovastatin, and likely other statins, through compromising HMG-CoA reductase activity, reduced cellular cholesterol affluence and led to altered rigidity and fluidity of the jail cell membranes. The resultant membrane physical properties facilitated the internalization and subsequent deposition of cell surface ErbB2, which synergized with lapatinib or neratinib to elicit potent inhibitory furnishings on ErbB2-positive breast cancer growth both in vitro and in vivo (Fig. half-dozen).
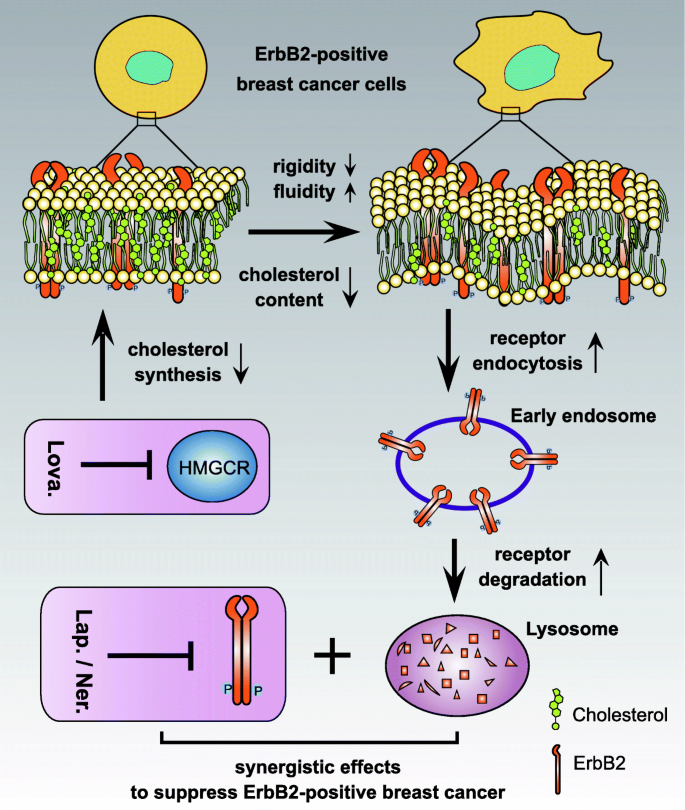
Schematic diagram depicting the synergistic effects of lovastatin with lapatinib. Through inhibiting HMGCR action, lovastatin reduces cellular cholesterol contents and hence alters the concrete properties of the jail cell membrane, conferring increased fluidity but decreased rigidity. These changes facilitates the internalization of prison cell surface ErbB2, which leads to its intracellular degradation through endosome to lysosome sorting. Reduced cellular ErbB2 expression sensitizes ErbB2-positive breast cancer cells to ErbB2 kinase inhibitors exemplified past lapatinib and neratinib, which collectively arm-twist more potent anti-cancer effects
Conclusions
ErbB2 has proven as a superlative therapeutic target in the clinical treatment of chest cancer. In the nowadays study, we report that the cholesterol content in cell membranes regulates the surface levels of ErbB2 via affecting membrane rigidity and fluidity. Cholesterol-lowering drugs exemplified past lovastatin potentiated the inhibitory effects of ErbB2 kinase inhibitors past down regulating ErbB2 expression. Therefore, our findings warrant farther investigations to reveal the synergistic effects of the statin class of medications with tyrosine kinase inhibitors against ErbB2 in the clinical direction of ErbB2-positive breast cancers.
Abbreviations
- EGFR:
-
Epidermal Growth Factor Receptor
- HMGCR:
-
HMG-CoA reductase (3-hydroxy-iii-methyl-glutaryl-coenzyme A reductase
- Lap:
-
Lapatinib
- Lova:
-
Lovastatin
- Ner:
-
Neratinib
- OA:
-
Oleic Acrid
- PBS:
-
Phosphate-Buffered Saline
- SEM:
-
Standard Error of the Hateful
References
-
Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Jail cell Biol. 2006;vii:505–16.
-
Jones RB, Gordus A, Krall JA, MacBeath Thousand. A quantitative poly peptide interaction network for the ErbB receptors using protein microarrays. Nature. 2006;439:168–74.
-
Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Jail cell Biol. 2001;2:127–37.
-
Wen Y, Hu MC, Makino K, Spohn B, Bartholomeusz One thousand, Yan DH, Hung MC. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res. 2000;60:6841–five.
-
Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer. 2012;12:553–63.
-
Kumler I, Tuxen MK, Nielsen DL. A systematic review of dual targeting in HER2-positive breast cancer. Cancer Treat Rev. 2014;40:259–70.
-
Rimawi MF, Schiff R, Osborne CK. Targeting HER2 for the treatment of breast cancer. Annu Rev Med. 2015;66:111–28.
-
Singh H, Walker AJ, Amiri-Kordestani L, Cheng J, Tang S, Balcazar P, Barnett-Ringgold K, Palmby TR, Cao X, Zheng Due north, Liu Q, Yu J, Pierce WF, Daniels SR, Sridhara R, Ibrahim A, Kluetz PG, Blumenthal GM, Beaver JA, Pazdur R. U.S. Food and Drug Assistants blessing: Neratinib for the extended adjuvant handling of early-phase HER2-positive breast Cancer. Clin Cancer Res. 2018;24:3486–91.
-
Nagy P, Vereb G, Sebestyen Z, Horvath G, Lockett SJ, Damjanovich S, Park JW, Jovin TM, Szollosi J. Lipid rafts and the local density of ErbB proteins influence the biological role of man- and heteroassociations of ErbB2. J Cell Sci. 2002;115:4251–62.
-
Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol. 1998;141:929–42.
-
Ringerike T, Blystad FD, Levy FO, Madshus IH, Stang E. Cholesterol is of import in command of EGF receptor kinase activity merely EGF receptors are not concentrated in caveolae. J Cell Sci. 2002;115:1331–40.
-
Simons Thou, Vaz WL. Model systems, lipid rafts, and prison cell membranes. Annu Rev Biophys Biomol Struct. 2004;33:269–95.
-
Holowka D, Gosse JA, Hammond AT, Han 10, Sengupta P, Smith NL, Wagenknecht-Wiesner A, Wu M, Young RM, Baird B. Lipid segregation and IgE receptor signaling: a decade of progress. Biochim Biophys Acta. 2005;1746:252–nine.
-
Nagy P, Jenei A, Damjanovich Southward, Jovin TM, Szolosi J. Complexity of signal transduction mediated by ErbB2: clues to the potential of receptor-targeted cancer therapy. Pathol Oncol Res. 1999;v:255–71.
-
Garcia JJ, Martinez-Ballarin E, Millan-Plano S, Allue JL, Albendea C, Fuentes L, Escanero JF. Furnishings of trace elements on membrane fluidity. J Trace Elem Med Biol. 2005;19:19–22.
-
Lingwood D, Simons K. Lipid rafts every bit a membrane-organizing principle. Science. 2010;327:46–50.
-
Chapman D. Phase transitions and fluidity characteristics of lipids and cell membranes. Q Rev Biophys. 1975;8:185–235.
-
Jaipuria Yard, Ukmar-Godec T, Zweckstetter M. Challenges and approaches to understand cholesterol-binding bear upon on membrane protein function: an NMR view. 2018;75:2137–51.
-
Rone MB, Fan J, Papadopoulos V. Cholesterol transport in steroid biosynthesis: part of poly peptide-protein interactions and implications in disease states. Biochim Biophys Acta. 2009;1791:646–58.
-
Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nat Rev Mol Jail cell Biol. 2008;9:125–38.
-
Borochov H, Shinitzky Yard. Vertical displacement of membrane proteins mediated by changes in microviscosity. Proc Natl Acad Sci U S A. 1976;73:4526–30.
-
Borochov H, Abbott RE, Schachter D, Shinitzky M. Modulation of erythrocyte membrane proteins past membrane cholesterol and lipid fluidity. Biochemistry. 1979;xviii:251–5.
-
Ohvo-Rekila H, Ramstedt B, Leppimaki P, Slotte JP. Cholesterol interactions with phospholipids in membranes. Prog Lipid Res. 2002;41:66–97.
-
Koshy C, Ziegler C. Structural insights into functional lipid-poly peptide interactions in secondary transporters. Biochim Biophys Acta. 2015;1850:476–87.
-
Citri A, Alroy I, Lavi S, Rubin C, Xu W, Grammatikakis N, Patterson C, Neckers L, Fry DW, Yarden Y. Drug-induced ubiquitylation and degradation of ErbB receptor tyrosine kinases: implications for cancer therapy. EMBO J. 2002;21:2407–17.
-
Pust Southward, Klokk TI, Musa N, Jenstad M, Risberg B, Erikstein B, Tcatchoff 50, Liestol K, Danielsen HE, van Deurs B, Sandvig One thousand. Flotillins as regulators of ErbB2 levels in breast cancer. Oncogene. 2013;32:3443–51.
-
Asp Due north, Pust Southward, Sandvig K. Flotillin depletion affects ErbB protein levels in different human being chest cancer cells. Biochim Biophys Acta. 2014;1843:1987–96.
-
Pereira PMR, Sharma SK, Carter LM, Edwards KJ, Pourat J, Ragupathi A, Janjigian YY, Durack JC, Lewis JS. Caveolin-i mediates cellular distribution of HER2 and affects trastuzumab binding and therapeutic efficacy. Nat Commun. 2018;9:5137.
-
Wang T, Zhang J, Wang S, Sunday Ten, Wang D, Gao Y, Zhang Y, Xu Fifty, Wu Y, Wu Y, Liu F, Liu Ten, Liu S, Zhang Y, Wang Y, Zou Fifty, Liu H. The exon xix-deleted EGFR undergoes ubiquitylation-mediated endocytic degradation via dynamin activity-dependent and -independent mechanisms. 2018;16:40.
-
Alvares SM, Dunn CA, Brown TA, Wayner EE, Carter WG. The role of membrane microdomains in transmembrane signaling through the epithelial glycoprotein Gp140/CDCP1. Biochim Biophys Acta. 2008;1780:486–96.
-
Maxfield FR, Tabas I. Function of cholesterol and lipid organization in disease. Nature. 2005;438:612–21.
-
Subczynski WK, Pasenkiewicz-Gierula M, Widomska J, Mainali L, Raguz Thousand. High cholesterol/low cholesterol: effects in biological membranes: a review. Cell Biochem Biophys. 2017;75:369–85.
-
Zhao W, Prijic S, Urban BC, Tisza MJ, Zuo Y, Li L, Tan Z, Chen X, Mani SA, Chang JT. Candidate Antimetastasis drugs suppress the metastatic chapters of breast Cancer cells by reducing membrane fluidity. Cancer Res. 2016;76:2037–49.
-
Zhang Y, Zhang J, Liu C, Du Due south, Feng L, Luan X, Zhang Y, Shi Y, Wang T, Wu Y, Cheng West, Meng South, Li M, Liu H. Neratinib induces ErbB2 ubiquitylation and endocytic degradation via HSP90 dissociation in breast cancer cells. Cancer Lett. 2016;382:176–85.
-
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30.
-
Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, Davies S, Fauron C, He X, Hu Z, Quackenbush JF, Stijleman IJ, Palazzo J, Marron JS, Nobel AB, Mardis Eastward, Nielsen TO, Ellis MJ, Perou CM, Bernard PS. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–7.
-
Stern HM. Improving treatment of HER2-positive cancers: opportunities and challenges. Sci Transl Med. 2012;4:127rv122.
-
Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni Fifty. Treatment of HER2-positive breast cancer: current condition and futurity perspectives. Nat Rev Clin Oncol. 2011;9:16–32.
-
Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Scientific discipline. 1972;175:720–31.
-
Ghosh-Choudhury Due north, Mandal CC, Ghosh-Choudhury N, Ghosh Choudhury Chiliad. Simvastatin induces derepression of PTEN expression via NFkappaB to inhibit chest cancer cell growth. Cell Signal. 2010;22:749–58.
-
Chowdhury K, Sharma A, Sharma T, Kumar Southward, Mandal CC. Simvastatin and MBCD inhibit breast Cancer-induced osteoclast activity by targeting Osteoclastogenic factors. Cancer Investig. 2017;35:403–13.
-
Shibata MA, Ito Y, Morimoto J, Otsuki Y. Lovastatin inhibits tumor growth and lung metastasis in mouse mammary carcinoma model: a p53-independent mitochondrial-mediated apoptotic mechanism. Carcinogenesis. 2004;25:1887–98.
-
Klawitter J, Shokati T, Moll V, Christians U, Klawitter J. Effects of lovastatin on breast cancer cells: a proteo-metabonomic report. Chest Cancer Res. 2010;12:R16.
-
Song 50, Tao X, Lin L, Chen C, Yao H, He G, Zou G, Cao Z, Yan Due south, Lu L, Yi H, Wu D, Tan Southward, Ouyang W, Dai Z. Cerasomal lovastatin nanohybrids for efficient inhibition of triple-negative breast cancer stem cells to meliorate therapeutic efficacy. ACS Appl Mater Interfaces. 2018;x:7022–30.
-
Efimova EV, Ricco N, Labay E, Mauceri HJ, Flor AC, Ramamurthy A, Sutton HG, Weichselbaum RR, Kron SJ. HMG-CoA reductase inhibition delays Dna repair and promotes senescence after tumor irradiation. Mol Cancer Ther. 2018;17:407–18.
Acknowledgements
The authors are grateful to the National Natural Science Foundation of China for fiscal supports.
Funding
This piece of work was supported by the National Natural Scientific discipline Foundation of Cathay (No. 81702628 to YZ and No. 81301901 to HL).
Availability of data and materials
All data were generated or analyzed during this study are included in this published commodity.
Writer information
Authors and Affiliations
Contributions
Study pattern: YZ and HL; Data Collection: JZ, QL, YG, DW, LX, YZ, SW, TW, FL, and MYZ; Data assay: JZ, QL, SH, HL, LZ, YZ, SL, KZ; Manuscript training: JZ and HL. All authors read and approved the concluding manuscript.
Corresponding authors
Ethics declarations
Authors' information
Not applicable.
Ethics blessing
Animal experiments were performed in accord with the 1996 National Institutes of Health Guide for the Care and apply of Laboratory Animals, and the procedures were approved by the Institutional Animal Intendance and Use Commission of Dalian Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution four.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give advisable credit to the original author(south) and the source, provide a link to the Creative Eatables license, and indicate if changes were fabricated. The Creative Eatables Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zippo/one.0/) applies to the data made bachelor in this article, unless otherwise stated.
Reprints and Permissions
Well-nigh this commodity
Cite this article
Zhang, J., Li, Q., Wu, Y. et al. Cholesterol content in prison cell membrane maintains surface levels of ErbB2 and confers a therapeutic vulnerability in ErbB2-positive breast cancer. Prison cell Commun Signal 17, fifteen (2019). https://doi.org/10.1186/s12964-019-0328-four
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/s12964-019-0328-iv
Keywords
- ErbB2
- Breast cancer
- Cholesterol
- Membrane fluidity
- Membrane rigidity
- Lovastatin
Source: https://biosignaling.biomedcentral.com/articles/10.1186/s12964-019-0328-4
Posted by: colemangingaid.blogspot.com

0 Response to "What Does Cholesterol Do In Animal Cell Membranes"
Post a Comment